what happens to the volume when the temperature increases
11.ix: Charles'due south Law- Book and Temperature
- Page ID
- 47533
Learning Objectives
- Acquire and apply Charles'south Law.
Everybody enjoys the aroma and taste of freshly-baked bread. Information technology is light and fluffy as a effect of the activeness of yeast on sugar. The yeast converts the sugar to carbon dioxide, which at loftier temperatures causes the dough to expand. The end result is an enjoyable treat, especially when covered with melted butter.
Charles'due south Law
French physicist Jacques Charles (1746-1823) studied the effect of temperature on the volume of a gas at abiding pressure. Charles'southward Law states that the book of a given mass of gas varies directly with the accented temperature of the gas when pressure is kept abiding. The absolute temperature is temperature measured with the Kelvin calibration. The Kelvin scale must be used because cypher on the Kelvin scale corresponds to a consummate stop of molecular motion.
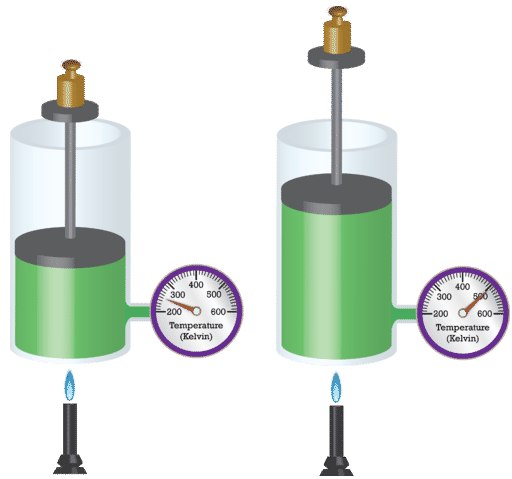
Mathematically, the direct relationship of Charles'south Police force can exist represented by the post-obit equation:
\[\frac{V}{T} = k\]
As with Boyle's Law, \(k\) is constant only for a given gas sample. The table beneath shows temperature and book data for a set amount of gas at a abiding force per unit area. The third column is the constant for this particular information set and is always equal to the volume divided by the Kelvin temperature.
| Temperature \(\left( \text{K} \right)\) | Book \(\left( \text{mL} \correct)\) | \(\frac{V}{T} = m\) \(\left( \frac{\text{mL}}{\text{K}} \right)\) |
|---|---|---|
| 50 | 20 | 0.40 |
| 100 | 40 | 0.twoscore |
| 150 | 60 | 0.twoscore |
| 200 | 80 | 0.40 |
| 300 | 120 | 0.twoscore |
| 500 | 200 | 0.40 |
| k | 400 | 0.twoscore |
When this data is graphed, the effect is a direct line, indicative of a direct relationship, shown in the figure below.
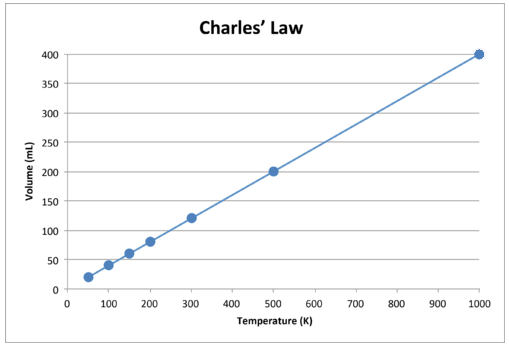
Find that the line goes exactly toward the origin, meaning that every bit the accented temperature of the gas approaches zero, its volume approaches zilch. Nonetheless, when a gas is brought to extremely cold temperatures, its molecules would eventually condense into the liquid state before reaching absolute cypher. The temperature at which this change into the liquid country occurs varies for different gases.
Charles'due south Police force can likewise exist used to compare changing conditions for a gas. Now we use \(V_1\) and \(T_1\) to stand for the initial volume and temperature of a gas, while \(V_2\) and \(T_2\) correspond the final volume and temperature. The mathematical relationship of Charles'southward Law becomes:
\[\frac{V_1}{T_1} = \frac{V_2}{T_2}\]
This equation can be used to calculate any one of the four quantities if the other three are known. The direct relationship will but concur if the temperatures are expressed in Kelvin. Temperatures in Celsius will not work. Recall the human relationship that \(\text{K} = \: ^\text{o} \text{C} + 273\).
Instance \(\PageIndex{i}\):
A balloon is filled to a volume of \(2.20 \: \text{Fifty}\) at a temperature of \(22^\text{o} \text{C}\). The balloon is then heated to a temperature of \(71^\text{o} \text{C}\). Detect the new volume of the airship.
Solution
| Steps for Trouble Solving | |
|---|---|
| Place the "given" data and what the problem is asking you to "observe." | Given: \(V_1 = 2.twenty \: \text{L}\) and \(T_1 = 22^\text{o} \text{C} = 295 \: \text{M}\) \(T_2 = 71^\text{o} \text{C} = 344 \: \text{Thou}\) Find: Five two = ? L |
| List other known quantities. | The temperatures accept first been converted to Kelvin. |
| Plan the trouble. | Start, rearrange the equation algebraically to solve for \(V_2\). \[V_2 = \frac{V_1 \times T_2}{T_1}\] |
| Cancel units and calculate. | Now substitute the known quantities into the equation and solve. \[V_2 = \frac{2.20 \: \text{L} \times 344 \: \cancel{\text{One thousand}}}{295 \: \cancel{\text{Thousand}}} = 2.57 \: \text{L}\] |
| Think about your issue. | The volume increases as the temperature increases. The event has three significant figures. |
Practice \(\PageIndex{1}\)
If V 1 = three.77 L and T 1 = 255 Thousand, what is 5 2 if T 2 = 123 K?
- Reply
-
1.82 L
Example \(\PageIndex{ii}\):
A sample of a gas has an initial volume of 34.viii 50 and an initial temperature of −67°C. What must be the temperature of the gas for its volume to be 25.0 Fifty?
Solution
| Steps for Trouble Solving | |
|---|---|
| Place the "given" information and what the problem is asking yous to "observe." | Given: Given: T 1 = -27oC and V 1 = 34.8 50 V two = 25.0 L Find: T 2 = ? K |
| List other known quantities. | G = -27oC + 273 |
| Plan the trouble. | one. Convert the initial temperature to Kelvin two. Rearrange the equation algebraically to solve for \(T_2\). \[T_2 = \frac{V_2 \times T_1}{V_1}\] |
| Cancel units and summate. | 1. −67°C + 273 = 206 Grand two. Substitute the known quantities into the equation and solve. \[T_2 = \frac{25.0 \: \abolish{\text{L}} \times 206 \: \text{Yard}}{34.8 \: \cancel{\text{50}}} = 148 \: \text{K}\] |
| Think well-nigh your result. | This is as well equal to −125°C. As temperature decreases, book decreases—which information technology does in this instance. |
Exercise \(\PageIndex{2}\)
If 5 ane = 623 mL, T ane = 255°C, and 5 ii = 277 mL, what is T ii ?
- Answer
-
235 M, or −38°C
Summary
- Charles'due south Law relates the volume and temperature of a gas at constant pressure level and corporeality.
Contributions & Attributions
This folio was constructed from content via the post-obit contributor(south) and edited (topically or extensively) by the LibreTexts development team to meet platform manner, presentation, and quality:
-
Marisa Alviar-Agnew (Sacramento City College)
-
Henry Agnew (UC Davis)
Source: https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Map:_Introductory_Chemistry_%28Tro%29/11:_Gases/11.09:_Charless_Law-_Volume_and_Temperature
0 Response to "what happens to the volume when the temperature increases"
Postar um comentário